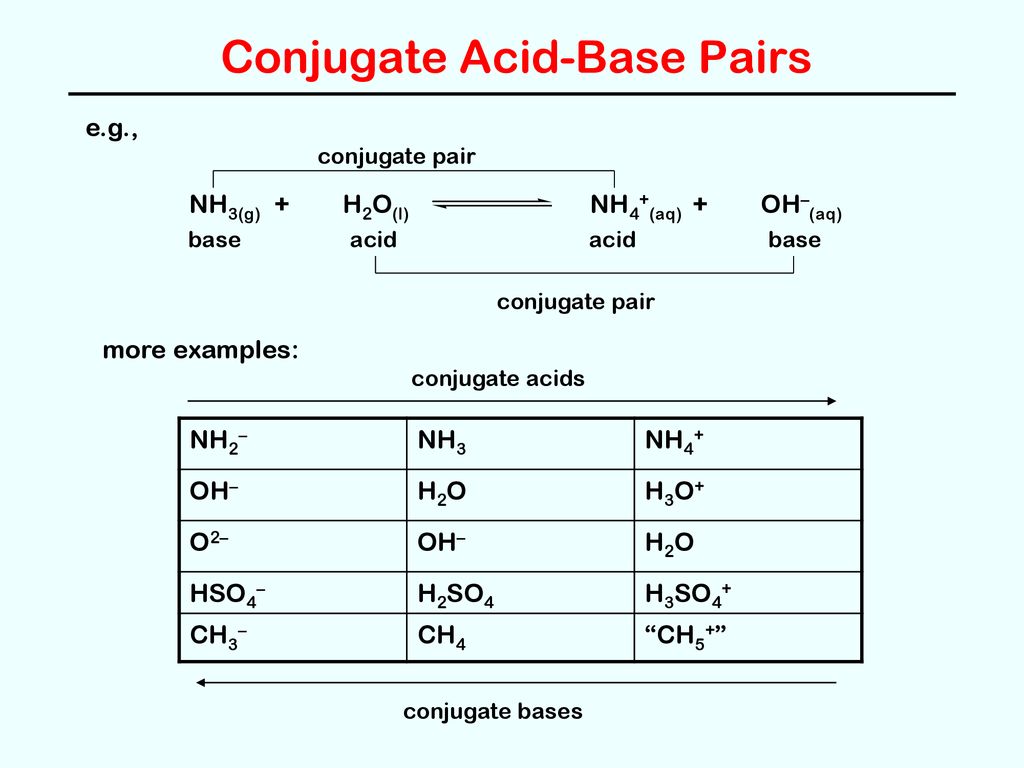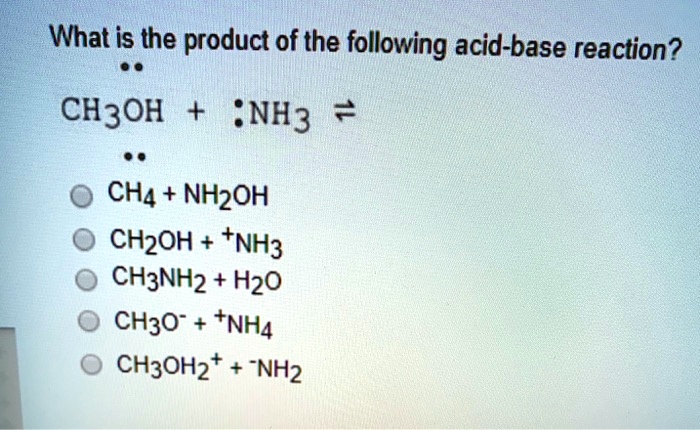
SOLVED: What is the product of the following acid-base reaction? CH3OH :NH3 2 CHA - NHZOH CH2OH +NH3 CH3NH2 Hzo CH3O" +NHA CH3OH2" NH2

Tröger's Base Polyimide Hybrid Membranes by Incorporating UiO-66-NH2 Nanoparticles for Gas Separation | Industrial & Engineering Chemistry Research

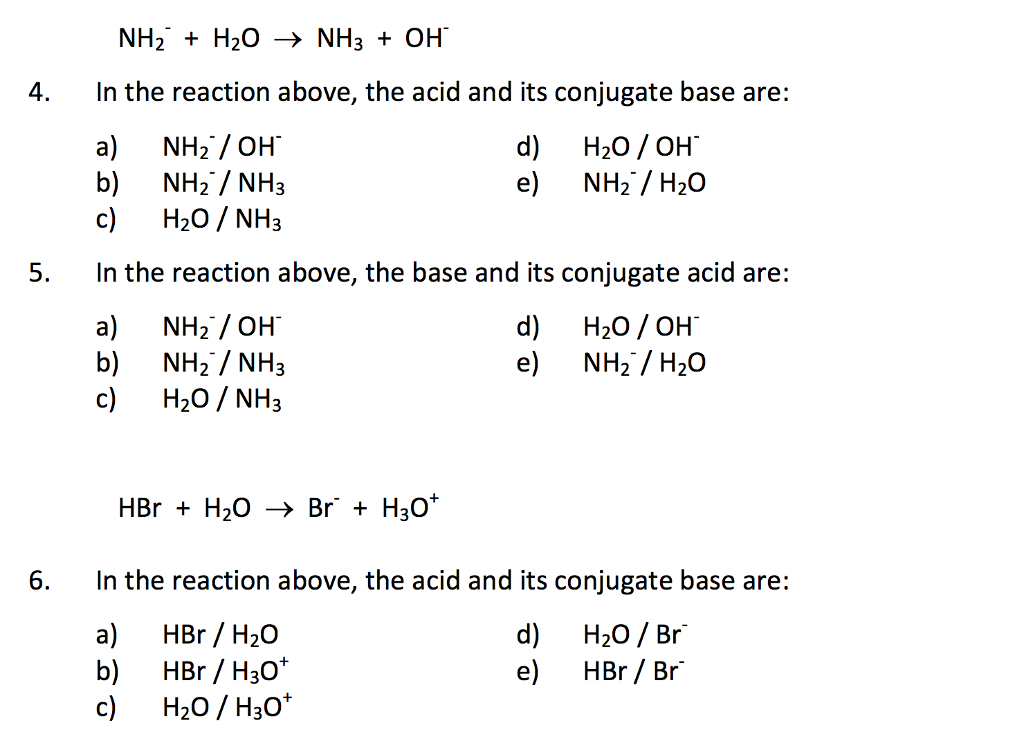
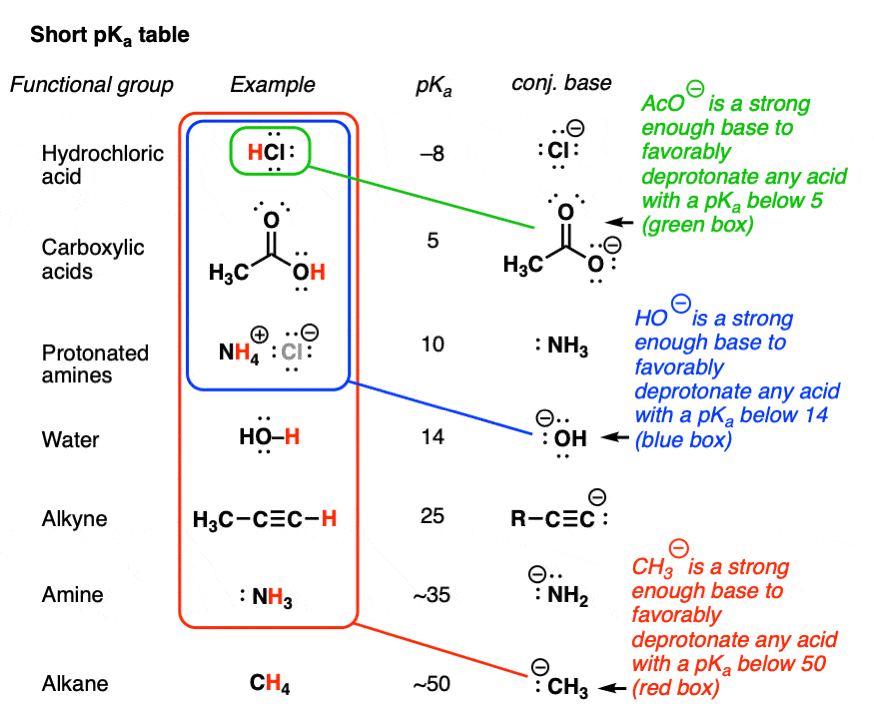
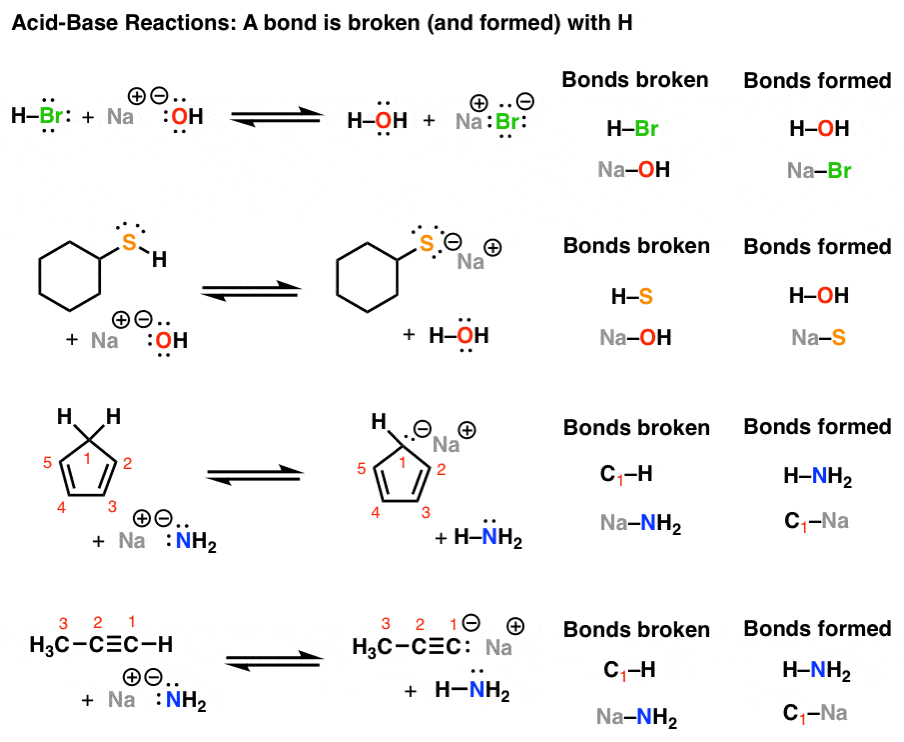
a) Mention conjugate base of each of the following: HS^-,H3O^+,H2PO4^-,HSO4^-,HF,CH3COOH,C6H5OH,HClO4,NH4^+ (b) Mention the conjugate acid of each of the following: OH^-,CH3COO^-,Cl^-,CO3^2 - ,H2PO4^-,CH3NH2,CH3COOH,NH2^- (c) Which of the following ...

Schiff base adduct pathway for reaction between -NH2 group of arginine... | Download Scientific Diagram
![CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is: CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:](https://dwes9vv9u0550.cloudfront.net/images/7494062/22483b28-e987-494e-85b6-898b531ad092.jpg)
CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:
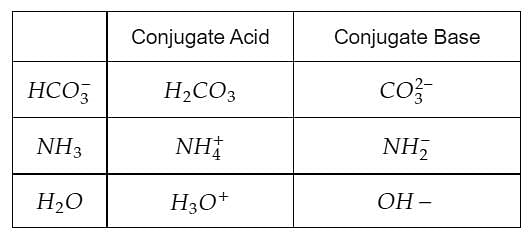
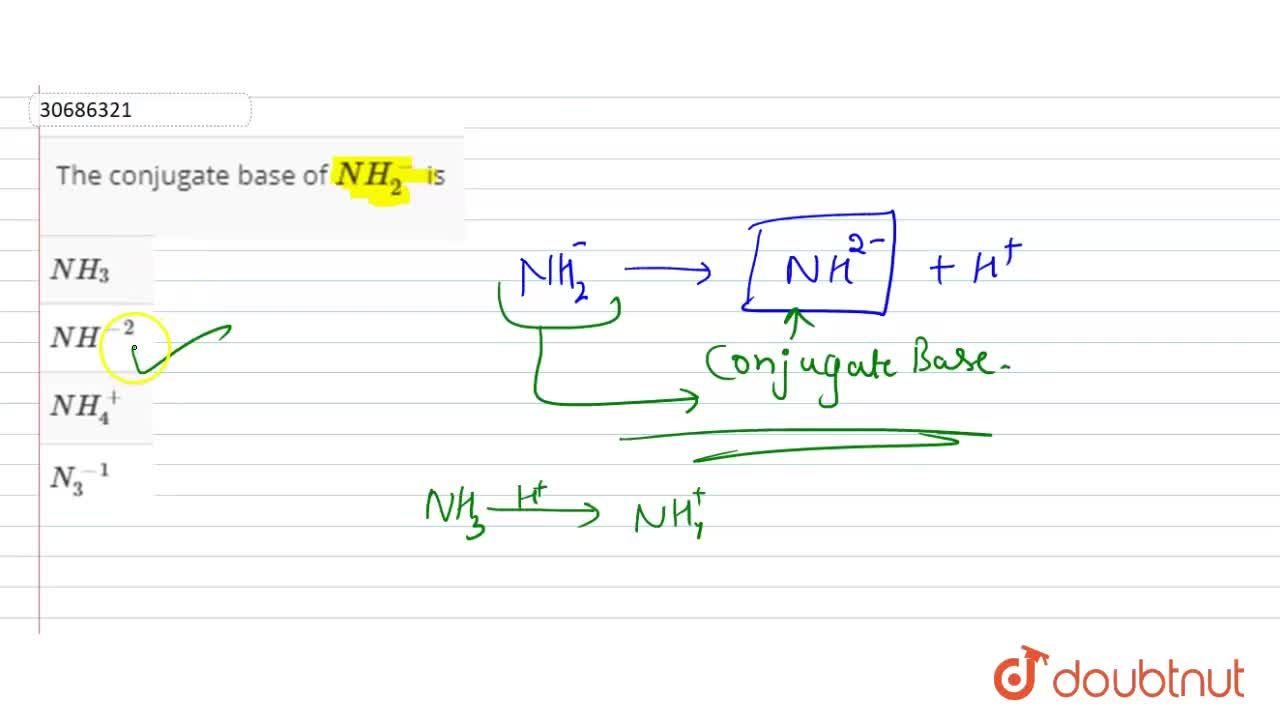
Which one of the following species can not act as both were bronsted acid and base? H2O NH2- NH3 HCO3-? | EduRev NEET Question

![Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ + Which is the correct for the following reaction: B (OH)3 + H2O → [B(OH)4]^ - + H^ +](https://dwes9vv9u0550.cloudfront.net/images/5674386/ee9fa7d9-c7ef-4a7f-90dd-f3bb61402c59.jpg)