![Effect of Oxygen on Ammonothermal Synthesis: Example of Na2[Zn(NH2)4] ⋅ (NH3)x and Na2[Zn(NH2)4] ⋅ (H2O)x - Kunkel - 2021 - European Journal of Inorganic Chemistry - Wiley Online Library Effect of Oxygen on Ammonothermal Synthesis: Example of Na2[Zn(NH2)4] ⋅ (NH3)x and Na2[Zn(NH2)4] ⋅ (H2O)x - Kunkel - 2021 - European Journal of Inorganic Chemistry - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/6d0a2979-5094-445c-a1c5-5dbc03a1765e/ejic202100721-toc-0001-m.jpg)
Effect of Oxygen on Ammonothermal Synthesis: Example of Na2[Zn(NH2)4] ⋅ (NH3)x and Na2[Zn(NH2)4] ⋅ (H2O)x - Kunkel - 2021 - European Journal of Inorganic Chemistry - Wiley Online Library
![Polynuclear complexes with bridging pyrophosphate ligands: synthesis and characterisation of {[(bipy)Cu(H2O)(μ-P2O7)Na2(H2O)6]·4H2O}, {[(bipy)Zn-(H2O)(μ-P2O7)Zn(bipy)]2·14H2O} and {[(bipy)(VO)2]2(μ-P2O7)]·5H2O} - Dalton Transactions (RSC Publishing) Polynuclear complexes with bridging pyrophosphate ligands: synthesis and characterisation of {[(bipy)Cu(H2O)(μ-P2O7)Na2(H2O)6]·4H2O}, {[(bipy)Zn-(H2O)(μ-P2O7)Zn(bipy)]2·14H2O} and {[(bipy)(VO)2]2(μ-P2O7)]·5H2O} - Dalton Transactions (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/B510752K)
Polynuclear complexes with bridging pyrophosphate ligands: synthesis and characterisation of {[(bipy)Cu(H2O)(μ-P2O7)Na2(H2O)6]·4H2O}, {[(bipy)Zn-(H2O)(μ-P2O7)Zn(bipy)]2·14H2O} and {[(bipy)(VO)2]2(μ-P2O7)]·5H2O} - Dalton Transactions (RSC Publishing)

Comparison of the Coordination of B12F122-, B12Cl122-, and B12H122- to Na+ in the Solid State: Crystal Structures and Thermal Behavior of Na2(B12F12), Na2(H2O)4(B12F12), Na2(B12Cl12), and Na2(H2O)6(B12Cl12). | Semantic Scholar
![Crystals | Free Full-Text | A Heterobimetallic 2-D Coordination Polymer [Na2 (Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture Crystals | Free Full-Text | A Heterobimetallic 2-D Coordination Polymer [Na2 (Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture](https://pub.mdpi-res.com/crystals/crystals-06-00096/article_deploy/html/images/crystals-06-00096-ag.png?1581089459)
Crystals | Free Full-Text | A Heterobimetallic 2-D Coordination Polymer [Na2 (Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D Supramolecular Architecture
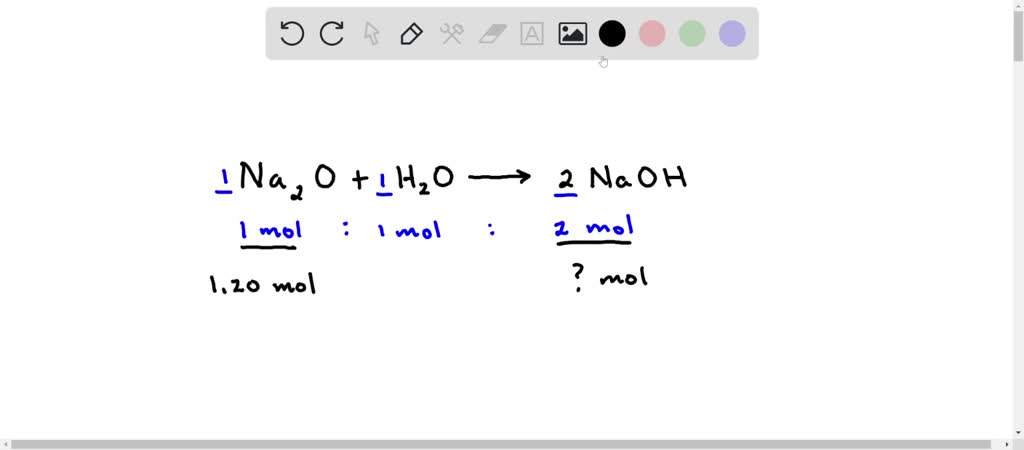
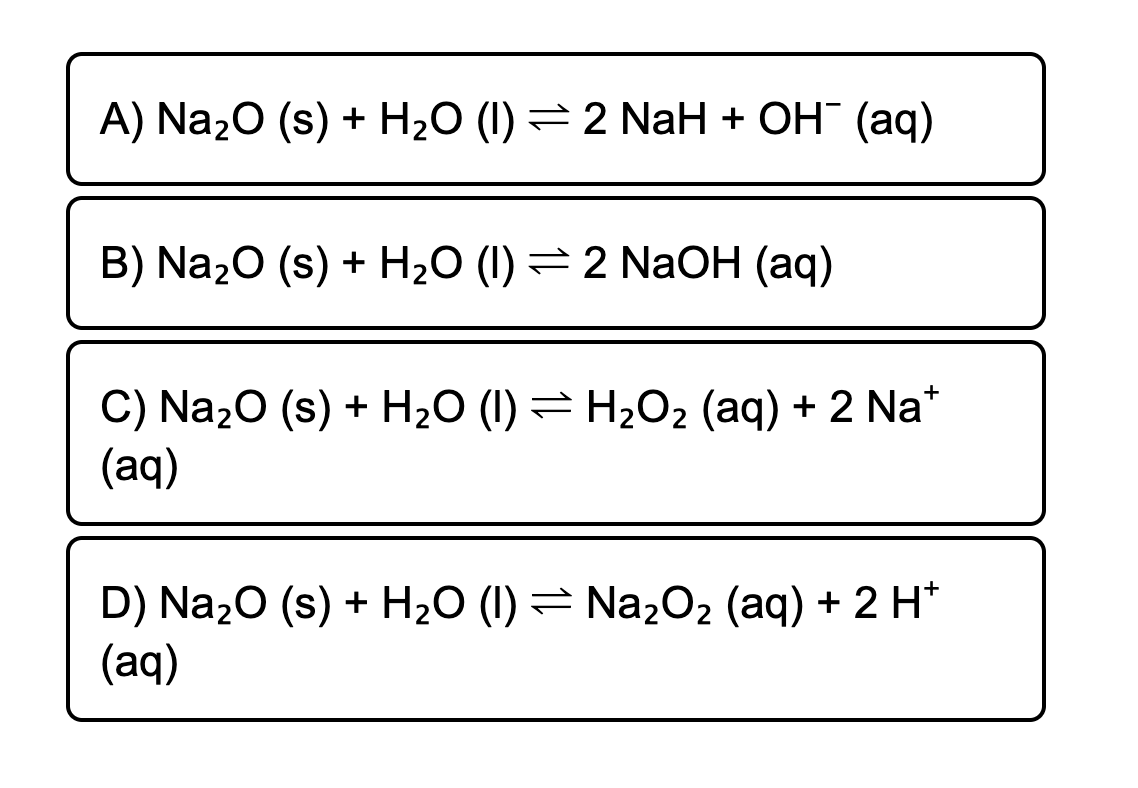
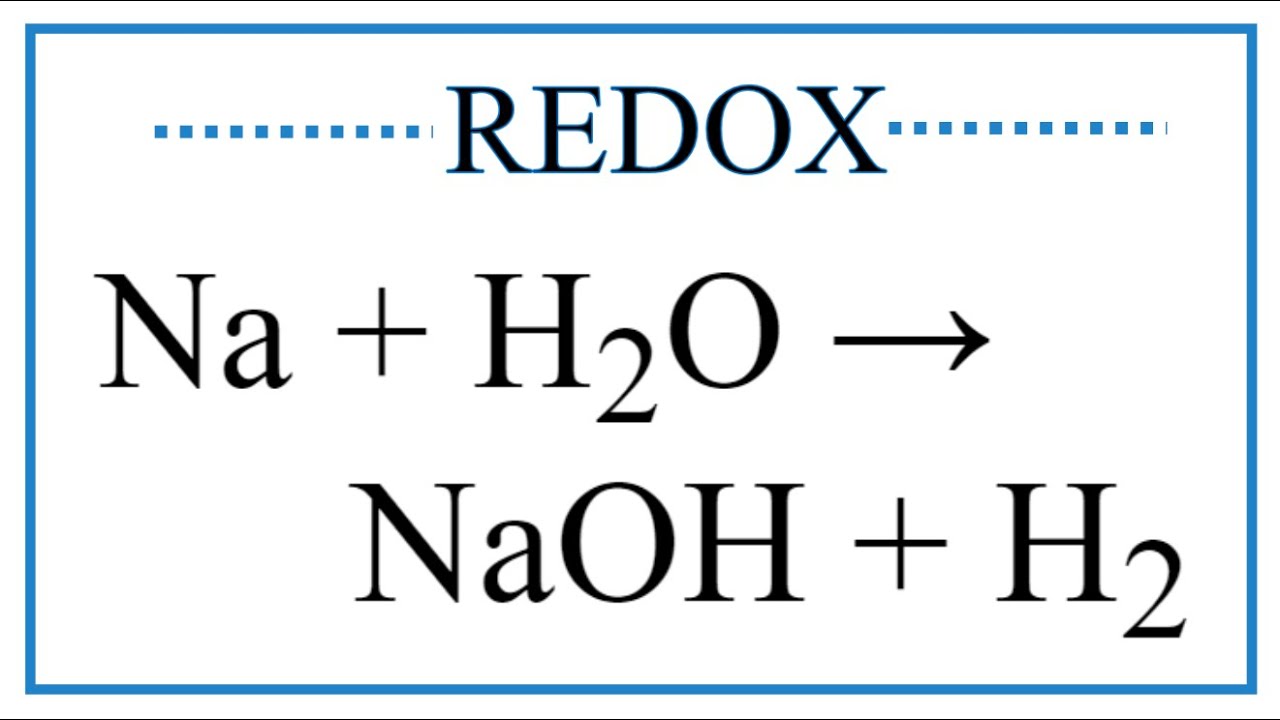
SOLVED: Given the following equation: Na2O + H2O —> 2 NaOH How many moles of NaOH are produced from 1.20 moles of Na2O?

EDTA Disodium salt solution for 1000 ml volumetric solution c(Na2-EDTA X 2 H2O) 0.1 mol/l (0.2N) - Th. Geyer

Comparison of the Coordination of B12F122-, B12Cl122-, and B12H122- to Na+ in the Solid State: Crystal Structures and Thermal Behavior of Na2(B12F12), Na2(H2O)4(B12F12), Na2(B12Cl12), and Na2(H2O)6(B12Cl12). | Semantic Scholar
![Synthesis, crystal structure, thermal, photoluminescent and magnetic properties of a new material: Na2[Ni(C2O4)2(H2O)2].6H2O - ScienceDirect Synthesis, crystal structure, thermal, photoluminescent and magnetic properties of a new material: Na2[Ni(C2O4)2(H2O)2].6H2O - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022286018311967-fx1.jpg)


![a) Exemplary structure of the complex Na2[Hf2(dpta)2] 7.5H2O .... | Download Scientific Diagram a) Exemplary structure of the complex Na2[Hf2(dpta)2] 7.5H2O .... | Download Scientific Diagram](https://www.researchgate.net/publication/352533926/figure/fig2/AS:1036268796379136@1624077142150/a-Exemplary-structure-of-the-complex-Na2Hf2dpta2-75H2O-05EtOH-b-2D-1-H-13-C.png)