MAN TGS 33.500 TGS 33.500 6x6 Euro 6 + MOL Kipper Oplegger Sattelzugmaschine kaufen Niederlande Heteren, YV27117

Gretchen Mol speaks with The Bare Magazine in NYC | The Notorious Bettie Page | Rounders — The Bare Magazine
Structure-based 3D-Pharmacophore modeling to discover novel interleukin 6 inhibitors: An in silico screening, molecular dynamics simulations and binding free energy calculations | PLOS ONE
A compound has a molar mass of approximately 180 g/mol and a percent composition of 40.00% Carbon, 6.72% Hydrogen, and 53.29% Oxygen. What is the molecular formula of the compound? - Quora
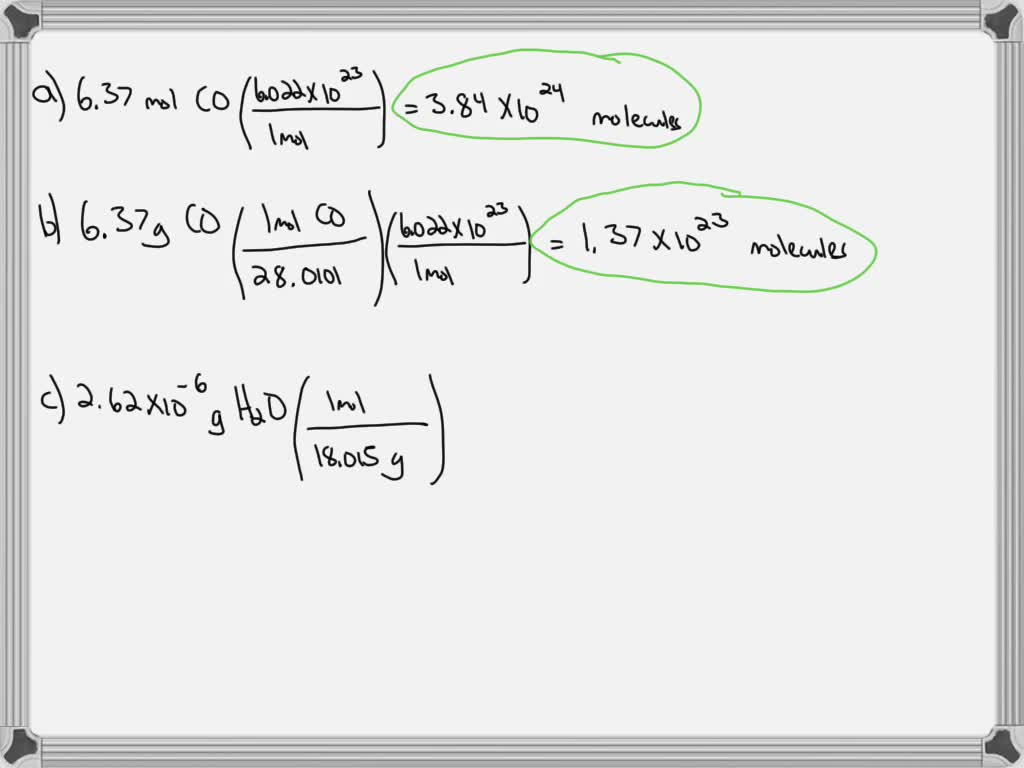
SOLVED: Calculate the number of molecules present in each of the following samples. a. 6.37 mol of carbon monoxide b. 6.37 g of carbon monoxide c. 2.62 × 10^-6 of water d.
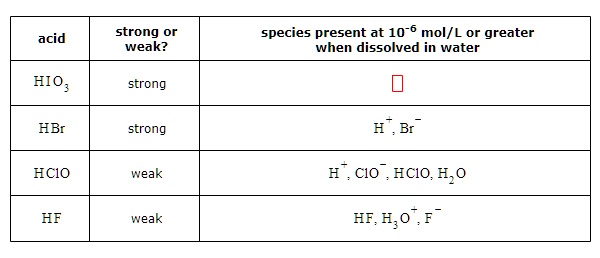
SOLVED: acid strong or weak? species present at 10-6 mol/L or greater when dissolved in water strong HBr strong H , Br HCIO weak #, Clo HClO, H,0 HE,H,0 ,F" HF weak