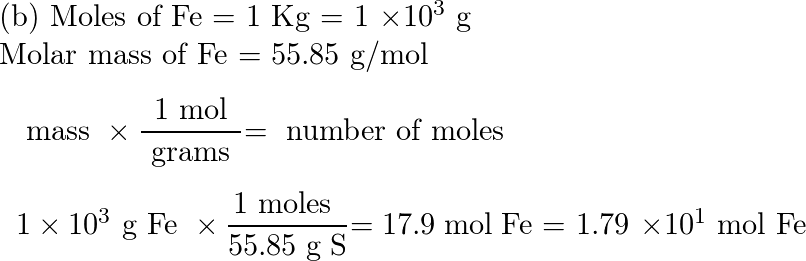![Ground and Excited States of the [Fe(H2O)6]2+ and [Fe(H2O)6]3+ Clusters: Insight into the Electronic Structure of the [Fe(H2O)6]2+–[Fe(H2O)6]3+ Complex | Journal of Chemical Theory and Computation Ground and Excited States of the [Fe(H2O)6]2+ and [Fe(H2O)6]3+ Clusters: Insight into the Electronic Structure of the [Fe(H2O)6]2+–[Fe(H2O)6]3+ Complex | Journal of Chemical Theory and Computation](https://pubs.acs.org/cms/10.1021/ct501143c/asset/images/medium/ct-2014-01143c_0017.gif)
Ground and Excited States of the [Fe(H2O)6]2+ and [Fe(H2O)6]3+ Clusters: Insight into the Electronic Structure of the [Fe(H2O)6]2+–[Fe(H2O)6]3+ Complex | Journal of Chemical Theory and Computation
Iron is produced by the reduction of iron (III) oxide using carbon monoxide. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g). How much Fe is produced from 1 kg of Fe2O3? - Quora

Fe(deferasirox)2: An Iron(III)-Based Magnetic Resonance Imaging T1 Contrast Agent Endowed with Remarkable Molecular and Functional Characteristics | Journal of the American Chemical Society
Ferrocene. Molecular model of the organometallic compound ferrocene (C10.H10.Fe). This is a metallocene consisting of two cyclopentadienyl rings bound by a central iron atom. The discovery of this compound and its properties

If 3.50 mol of Fe reacts with 3.00 mol of oxygen in the following reaction: 4 Fe(s) + 3 O2(g) → 2 - Brainly.com