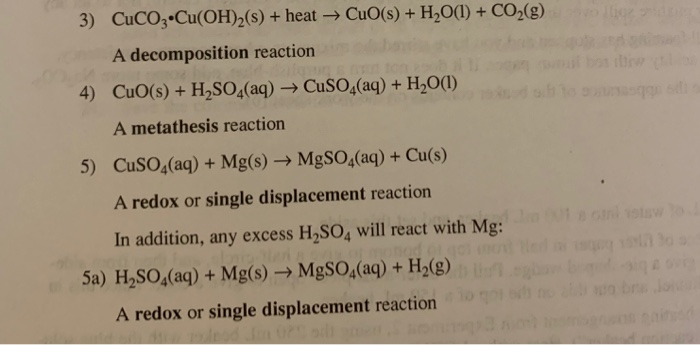Temperature contour with CuO-H2O (DI) as a working fluid in HCE at 40 LPH. | Download Scientific Diagram

Pushing the Limits of Rapid Anodic Growth of CuO/Cu(OH)2 Nanoneedles on Cu for the Methanol Oxidation Reaction: Anodization pH Is the Game Changer | ACS Applied Energy Materials

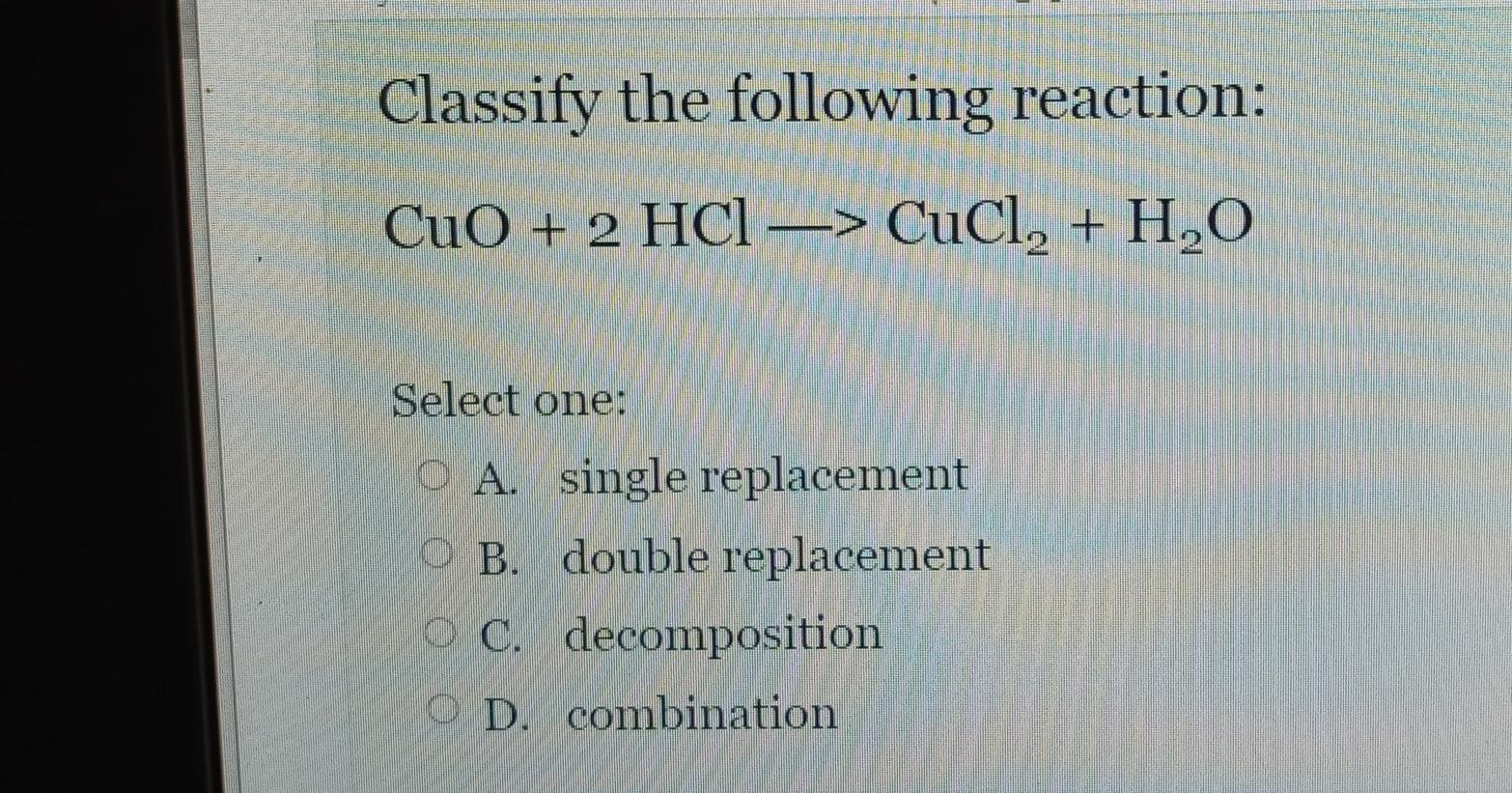
SOLVED: Which of the following is a double replacement reaction? 13 CuO+Hz => Cu+H2O 2 HBr + KOH => H2O + KBr 3. SO2 + HzO => HzSO3 4. 2HI => 12 + H2 5. Cu + AgNO3 => Cu(NO3h2 + Ag 0 5 0 3 4 2

Rotating flow of Ag-CuO/H2O hybrid nanofluid with radiation and partial slip boundary effects | SpringerLink
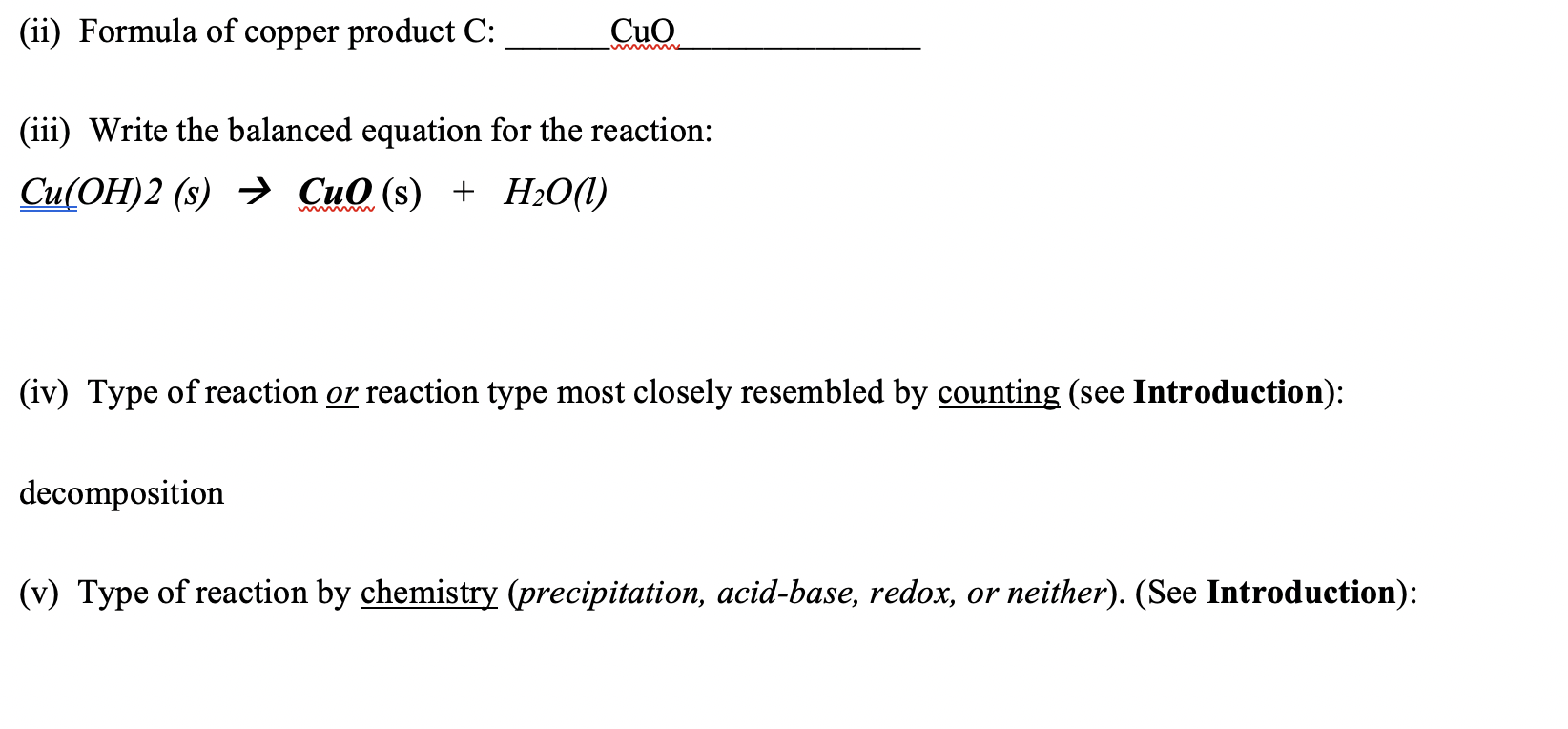
![SOLVED: ComeC: (C) Cu(OH)2(s) - > CuO(s) H2O() reaction type [decomposition redox reaction molecular: already given above total ionic chemPad Xox Greek Your answer contains an incorrect or incomplete chem] net ionic SOLVED: ComeC: (C) Cu(OH)2(s) - > CuO(s) H2O() reaction type [decomposition redox reaction molecular: already given above total ionic chemPad Xox Greek Your answer contains an incorrect or incomplete chem] net ionic](https://cdn.numerade.com/ask_images/b719cf4b6fcb402a984590a26f119066.jpg)