OneClass: 3. Consider the reaction: 2HCl(aq) + Ba(OH)2(a) â†' BaCl2(aq) + 2 H2O(l) Δ --118 kl A) Cal...
40. 50ml H2O is added to a 50ml solution of Ba(OH)2 of strenth 0.01M. The pH value of the resuultingsolution will be

SOLVED: Consider the unbalanced equation for the neutralization of acetic acid: HC2H3O2(aq) + Ba(OH)2(aq)-H2O(l ) + Ba(C2H3O2)2(aq) Balance the equation and determine how many moles of Ba(OH)2 are required to completely neutralize

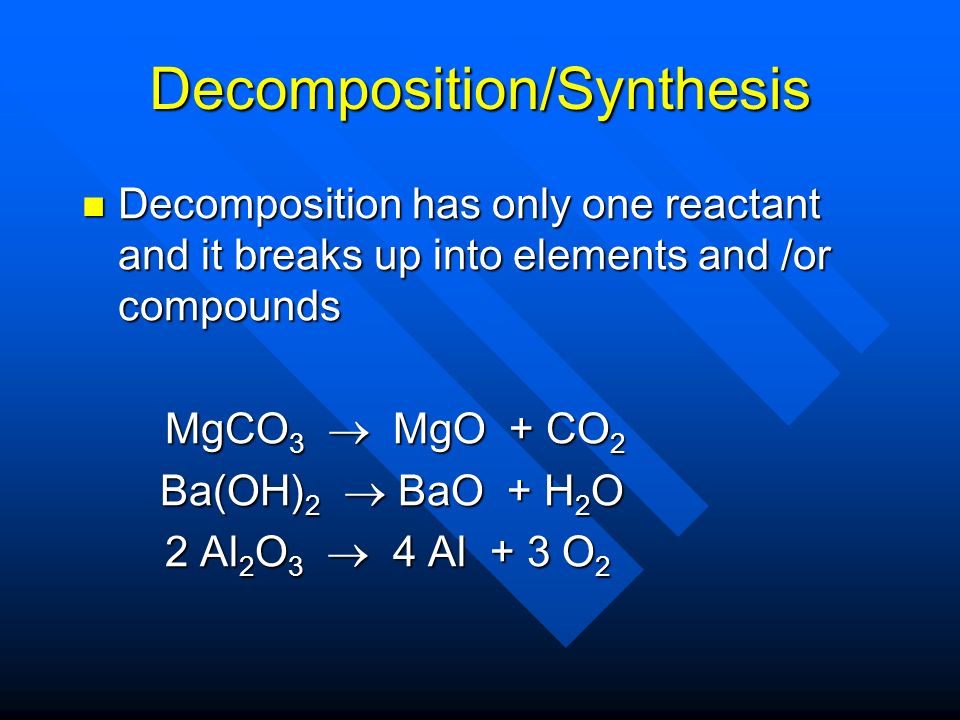
рассчитайте по термохимическому уравнению BaO+H2O=Ba(OH)2+73 кДж сколько выделяется энергии - Школьные Знания.com

PPT – Ba(OH)2.8H2O 2NH4SCN ? Ba(SCN)2 2NH3 10H2O PowerPoint presentation | free to download - id: 56f00c-ZjgyZ
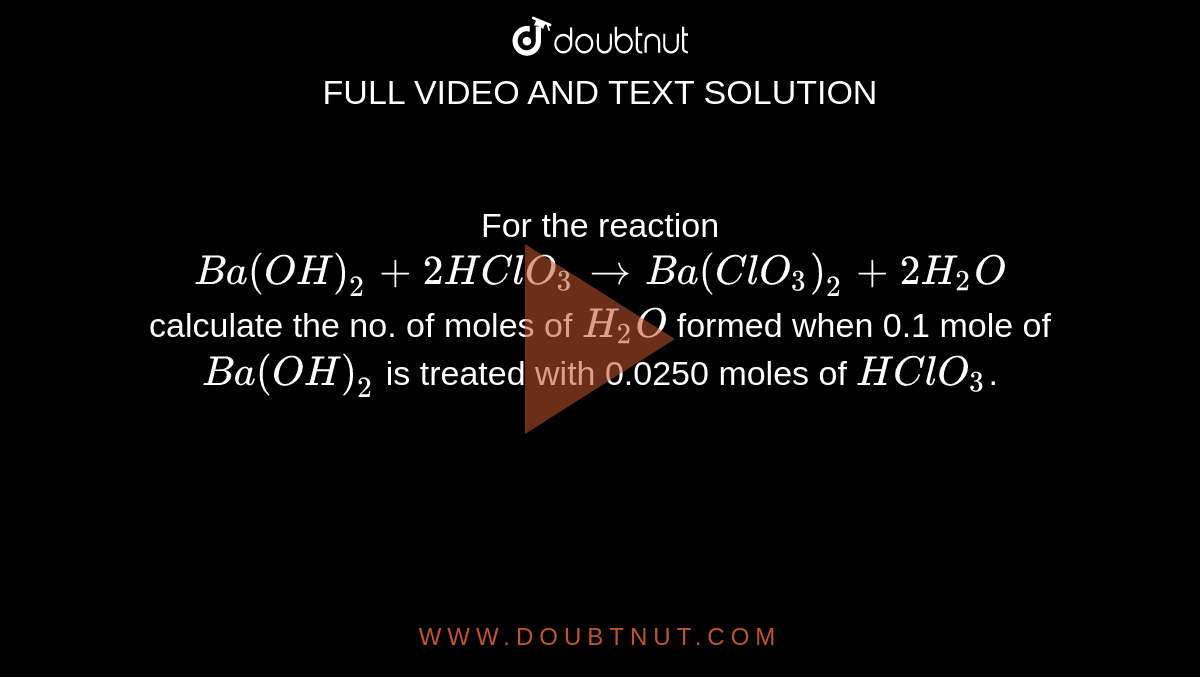
For the reaction Ba(OH)(2)+2HClO(3)rarr Ba(ClO(3))(2)+2H(2)O calculate the no. of moles of H(2)O formed when 0.1 mole of Ba(OH)(2) is treated with 0.0250 moles of HClO(3).
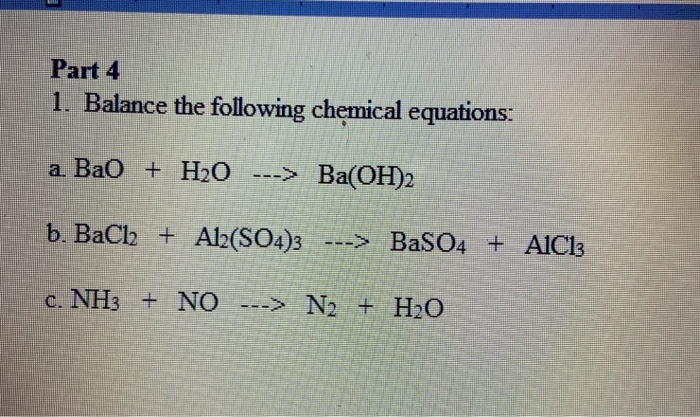
BALANCE THIS GIVEN EQUATION BY THE HELP OF THE ALTERNATE WAY OF BALANCING NOT THE TRADITIONAL WAY BY SHOWING THE APPROPRIATE STEPS ELABORATELY. EQN : Ba(OH)2 + NH4Cl —> BaCl2 + NH3 + H2O