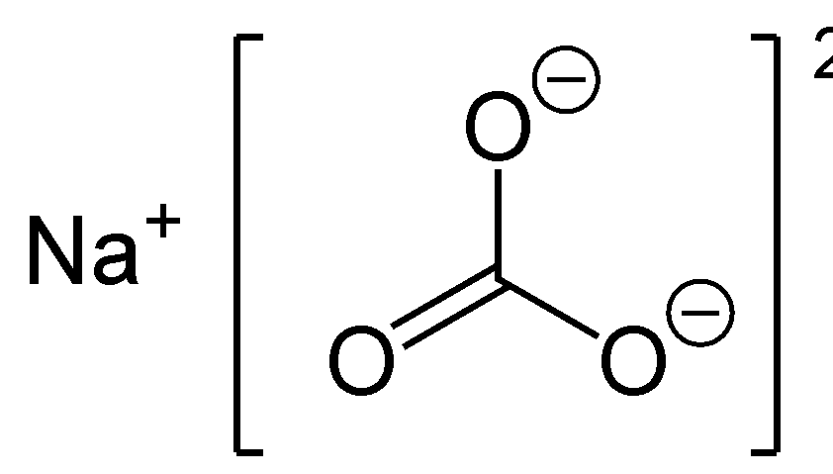
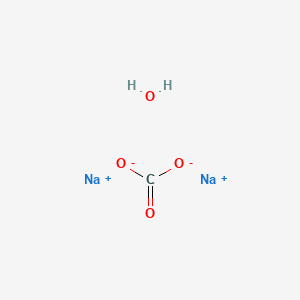
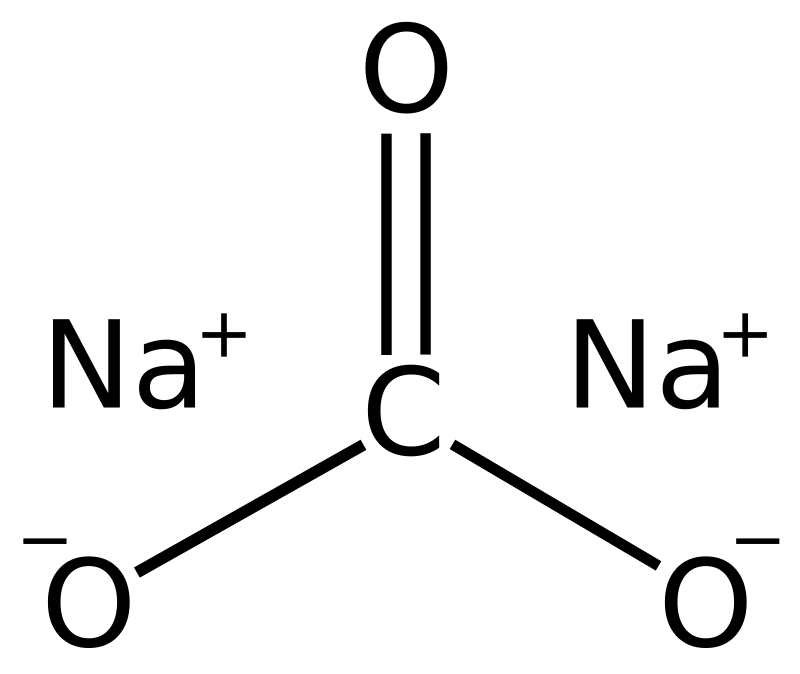
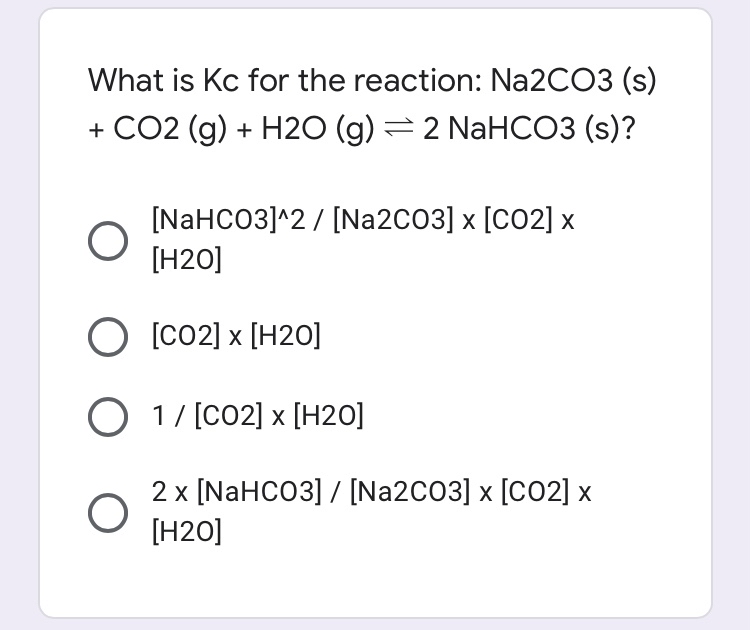A refinement of the crystal structure of Na2CO3 H2O - Journal of Research of NIST and Predecessor Publications - NIST Digital Archives

Phase Equilibria of the NaOH–NaBO2–Na2CO3–H2O System at 30 °C, 60 °C, and 100 °C | Journal of Chemical & Engineering Data
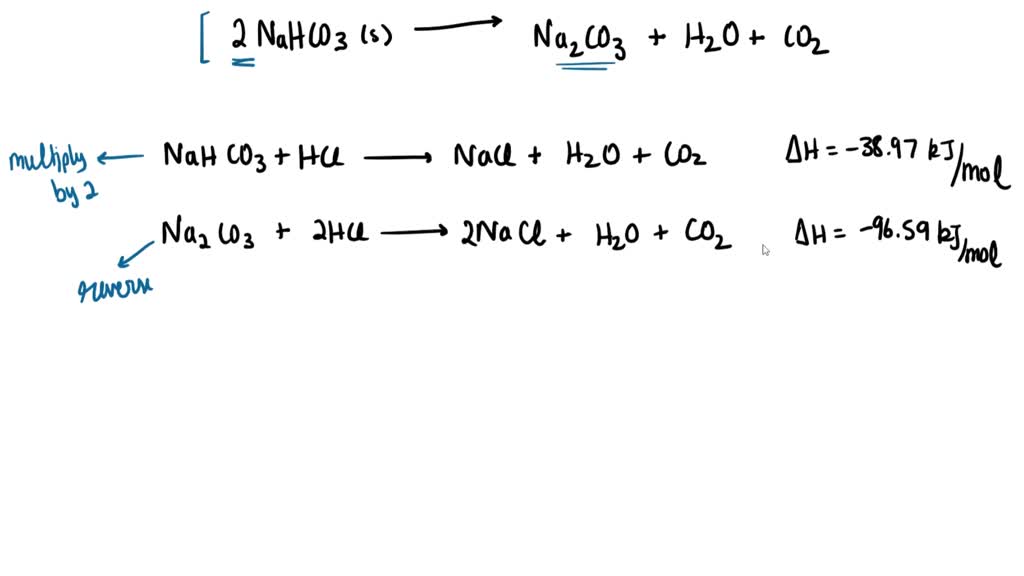
SOLVED: For the reaction Na2CO3 (s) + 2HCl(g) → NaCl(s) + CO2 (g) + H2O(l) ∆H is -144.1 kJ. What is ∆U.

Scheme 3. Reagents and conditions: a) H2O, Na2CO3, rt, 1 min; b) CH3CN,... | Download Scientific Diagram
Site-selective Suzuki–Miyaura coupling of heteroaryl halides – understanding the trends for pharmaceutically import